Kairos Pharma, Ltd. Common Stock (KAPA)
0.9179
-0.0221 (-2.35%)
NYSE · Last Trade: Jul 31st, 11:21 AM EDT
Detailed Quote
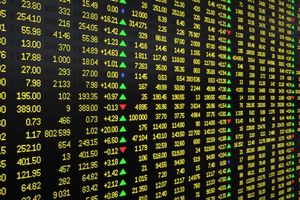
| Previous Close | 0.9400 |
|---|---|
| Open | 0.9500 |
| Bid | 0.9115 |
| Ask | 0.9242 |
| Day's Range | 0.8678 - 0.9500 |
| 52 Week Range | 0.4000 - 4.000 |
| Volume | 739,502 |
| Market Cap | 15.47M |
| PE Ratio (TTM) | -3.530 |
| EPS (TTM) | -0.3 |
| Dividend & Yield | N/A (N/A) |
| 1 Month Average Volume | 12,323,035 |
Chart
News & Press Releases

Via Benzinga · July 30, 2025

Via Benzinga · July 17, 2025

Via Benzinga · July 16, 2025

In today's session, these stocks are experiencing unusual volume.
Via Chartmill · July 16, 2025
InvestorNewsBreaks – Kairos Pharma Ltd. (NYSE American: KAPA) to Present at H.C. Wainwright Global Investment Conference
Kairos Pharma (NYSE American: KAPA), a clinical-stage biopharma company advancing cancer therapeutics, will participate in the H.C. Wainwright 27th Annual Global Investment Conference, scheduled for September 8–10, 2025, at the Lotte New York Palace Hotel in New York City. The company will host one-on-one meetings and deliver a presentation available virtually via kairospharma.com.
Via Investor Brand Network · July 16, 2025

Kairos Pharma, Ltd. (NYSE American:KAPA), a clinical-stage biopharmaceutical company focused on innovative cancer therapeutics, today announces participation and presentation at the H.C. Wainwright 27th Annual Global Investment Conference, taking place September 8–10, 2025, at the Lotte New York Palace Hotel in New York City. Kairos Pharma will participate in on-on-one meetings and present at the meeting which will be available virtually and hosted on the kairospharma.com website.
By Kairos Pharma, Ltd · Via Business Wire · July 16, 2025

Via Benzinga · July 16, 2025
Via Benzinga · July 16, 2025

Via Benzinga · July 15, 2025

The regular session of the US market on Tuesday is now over, but let's get a preview of the after-hours session and explore the top gainers and losers driving the post-market movements.
Via Chartmill · July 15, 2025

Momentum in medical and tech-focused small caps continues to explode, led by
Via AB Newswire · July 15, 2025
Kairos Pharma reported strong safety results from a Phase 2 trial of ENV-105 in mCRPC, with no serious toxicities seen in the first 10 patients.
Via Benzinga · July 15, 2025

Discover the most active stocks in Tuesday's session. Stay informed about the stocks that are generating the most trading volume!
Via Chartmill · July 15, 2025

Wondering how the US markets performed in the middle of the day on Tuesday? Discover the movers and shakers of today's session in our comprehensive analysis.
Via Chartmill · July 15, 2025

Via Benzinga · July 15, 2025

Let's have a look at what is happening on the US markets on Tuesday. Below you can find the gap up and gap down stocks in today's session.
Via Chartmill · July 15, 2025
BioMedNewsBreaks — Kairos Pharma Ltd. (NYSE American: KAPA) Reports Positive Safety Results in Phase 2 Study of Prostate Cancer Drug ENV-105
Kairos Pharma (NYSE American: KAPA), a clinical-stage biopharma company developing cancer therapies, announced favorable interim safety results from its ongoing Phase 2 trial of ENV-105 (carotuximab) in patients with metastatic castration-resistant prostate cancer. The first-in-class CD105 antagonist showed no dose-limiting toxicities or unexpected adverse events in the first ten patients, and no Grade 3 or 4 toxicities were observed. ENV-105 was well tolerated when combined with apalutamide, a standard hormone therapy. The 100-patient trial is underway at Cedars-Sinai, City of Hope, and Huntsman Cancer Center, with interim efficacy data expected in September 2025. Kairos plans to engage regulators on a potential Phase 3 study.
Via Investor Brand Network · July 15, 2025

Discover the top movers in Tuesday's pre-market session and stay informed about market dynamics.
Via Chartmill · July 15, 2025

Via Benzinga · July 15, 2025

Kairos Pharma, Ltd. (NYSE American:KAPA), a clinical-stage biopharmaceutical company focused on innovative cancer therapeutics, today announces positive safety results from its ongoing Phase 2 clinical trial of ENV-105 (carotuximab) in patients with metastatic castration-resistant prostate cancer (mCRPC). The interim safety analysis of the trial demonstrated that ENV-105, a first-in-class CD105 antagonist, was well tolerated when combined with standard of care hormone therapy, apalutamide, from the first ten enrolled patients. Thus far, there have been no dose-limiting toxicities or unexpected adverse events reported to date. In addition, the treatment-related side effects were manageable with standard supportive care. Notably, no Grade 3 or 4 toxicities were observed.
By Kairos Pharma, Ltd · Via Business Wire · July 15, 2025

Volume analysis on 2025-06-10: stocks with an unusual volume in today's session.
Via Chartmill · June 10, 2025

Via Benzinga · June 9, 2025

As the regular session of the US market concludes on Monday, let's get an insight into the after-hours session and identify the stocks leading the pack in terms of gains and losses.
Via Chartmill · June 9, 2025

